Platform Introduction
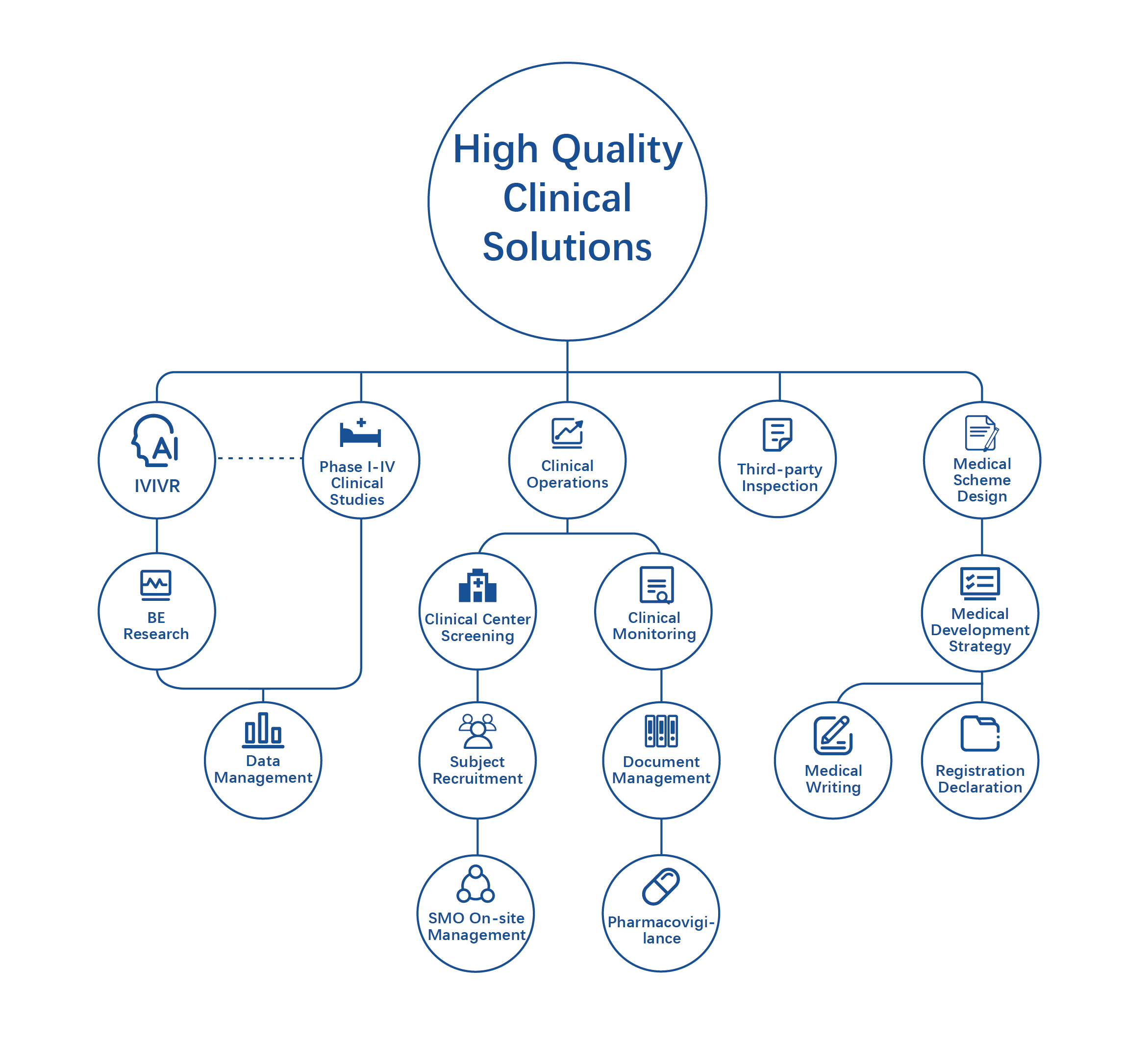
The Clinical Section (Deep Blue Sea · Zhengzhou) of Leadingpharm has 16 years of experience in clinical research and development. It has a clinical service team with more than 300 highly professional individuals. It includes the Medical Department, Clinical Operation Department, Data Department, Pharmacovigilance Department, Quality Control Committee, Drug Administration Department, Project Management Department, etc. Relying on Leadingpharm CXO Service System, as well as Leadingpharm’s innovative Interactive Clinical Service Model and the establishment of a special government guidance fund, Leadingpharm can provide innovative enterprises with investment and financing at different stages for high-quality projects, and help the rapid promotion of innovative projects. It can direct the design and development of medical scheme based on the clinical data of previous projects. Besides, based on a strong quantitative pharmacology and the IVIVR team, a series of studies can be conducted for specific diseases and specific populations to support the establishment of new models for clinical development of new drugs guided by models, support the scientific innovation of new drug registration, and ultimately improve the efficiency of clinical development of new drugs.
Begin with the end in mind, do a good job in top-level design,and be a full process partner in the creation of new drugs.
The Clinical Research Platform focuses on providing professional services and high-quality solutions covering the whole clinical process for global pharmaceutical innovation enterprises. With the help of rich industry experience, a vast network of clinical trial institutions, and a strong professional team, we have provided diversified research and development support and clinical technical services to more than 300 well-known domestic and foreign enterprises, covering biopharmaceuticals, chemical drugs, traditional Chinese medicine, cell and gene therapy, and overcoming key bottlenecks in the launch of new drugs.
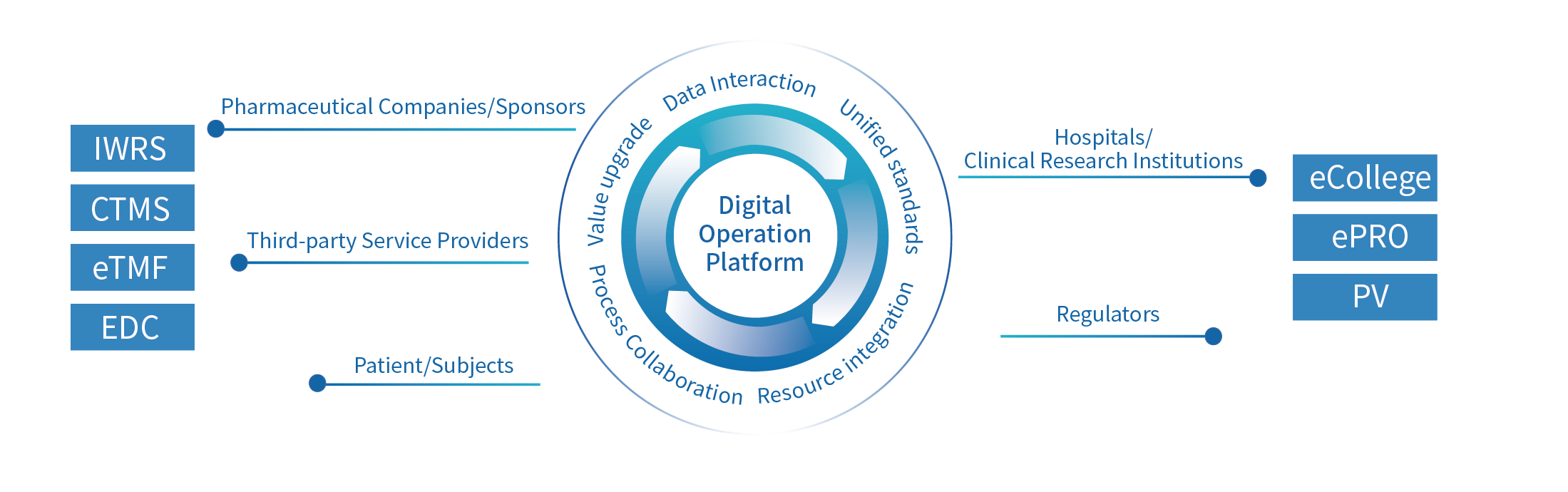
The Digital Operation Platform has realized all-round digital driven. By leveraging professional digital software, collaboration platforms, and professional service capabilities, the platform connects industry participants such as hospitals, pharmaceutical companies, service providers, regulatory agencies, and patients to achieve comprehensive data-driven approaches in all aspects. We are also committed to promoting the development of clinical research through innovative service models and multi-dimensional information technology.

 Hot line:010-83057670
Hot line:010-83057670
 EN
EN
 010-83057670
010-83057670 Address:
Address: Marketing Department:
Marketing Department: Leadingpharm
Leadingpharm Pharm News
Pharm News 010-61006450
010-61006450
