
——————————————————
The Analytical Testing Center can provide testing technical services to domestic and foreign pharmaceutical enterprises, and has accumulated rich experience in drug quality research, mainly providing research and testing services that meet the requirements of NMPA, FDA, EMA and other regulations, such as the analysis of trace substances, genotoxic impurities, element impurities, residual solvents, polymer impurities, complex preparation excipients, as well as drug contact system compatibility research. The Center has successively passed the ISO9001 Quality Management System Certification and the Laboratory Accreditation Qualification of China National Accreditation Service for Conformity Assessment(CNAS)Business Scope
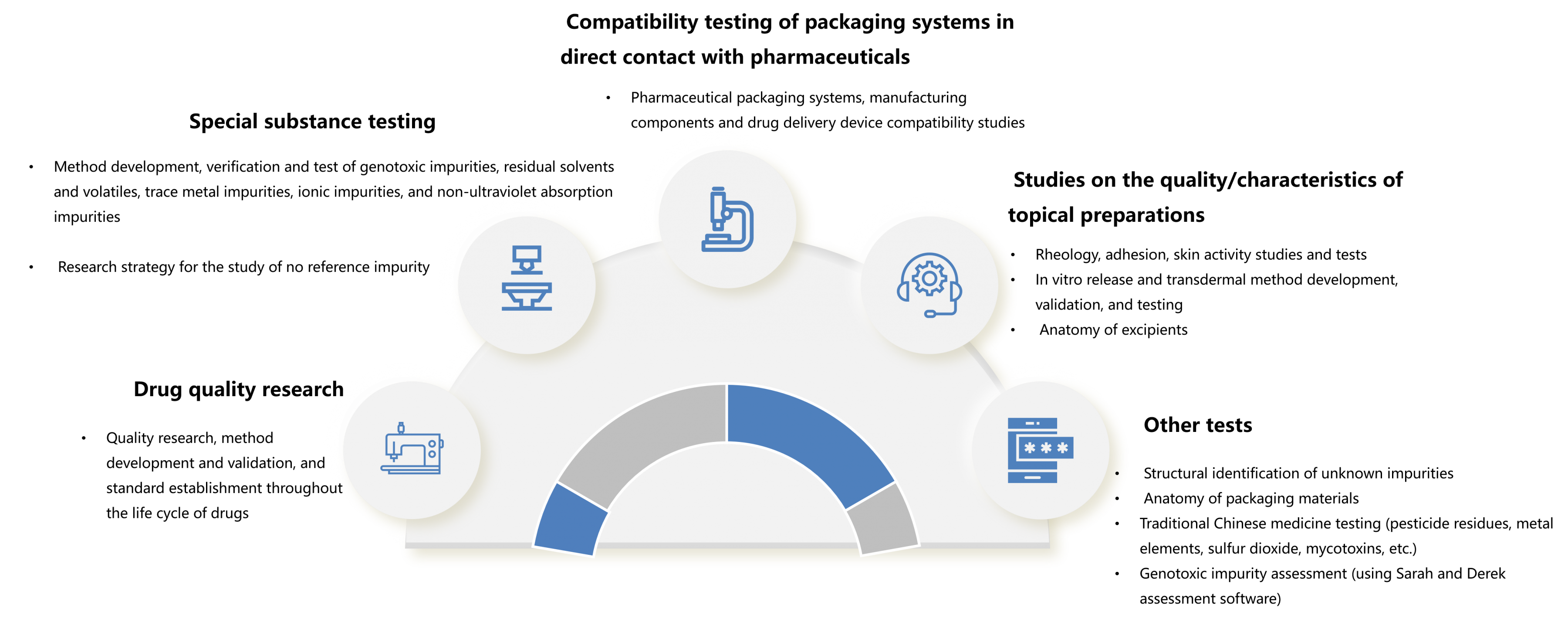
♦Based on advanced testing equipment and perfect quality management system of Leadingpharm, the Analytical Testing Center can help the project team complete the development of complex compound testing methods, compatibility research (packaging materials, drug delivery devices, production components, etc.) and the detection of special impurities (elemental impurities, genotoxic impurities, gold ions, polymers, etc.).
The main business areas of the Center are as follows:
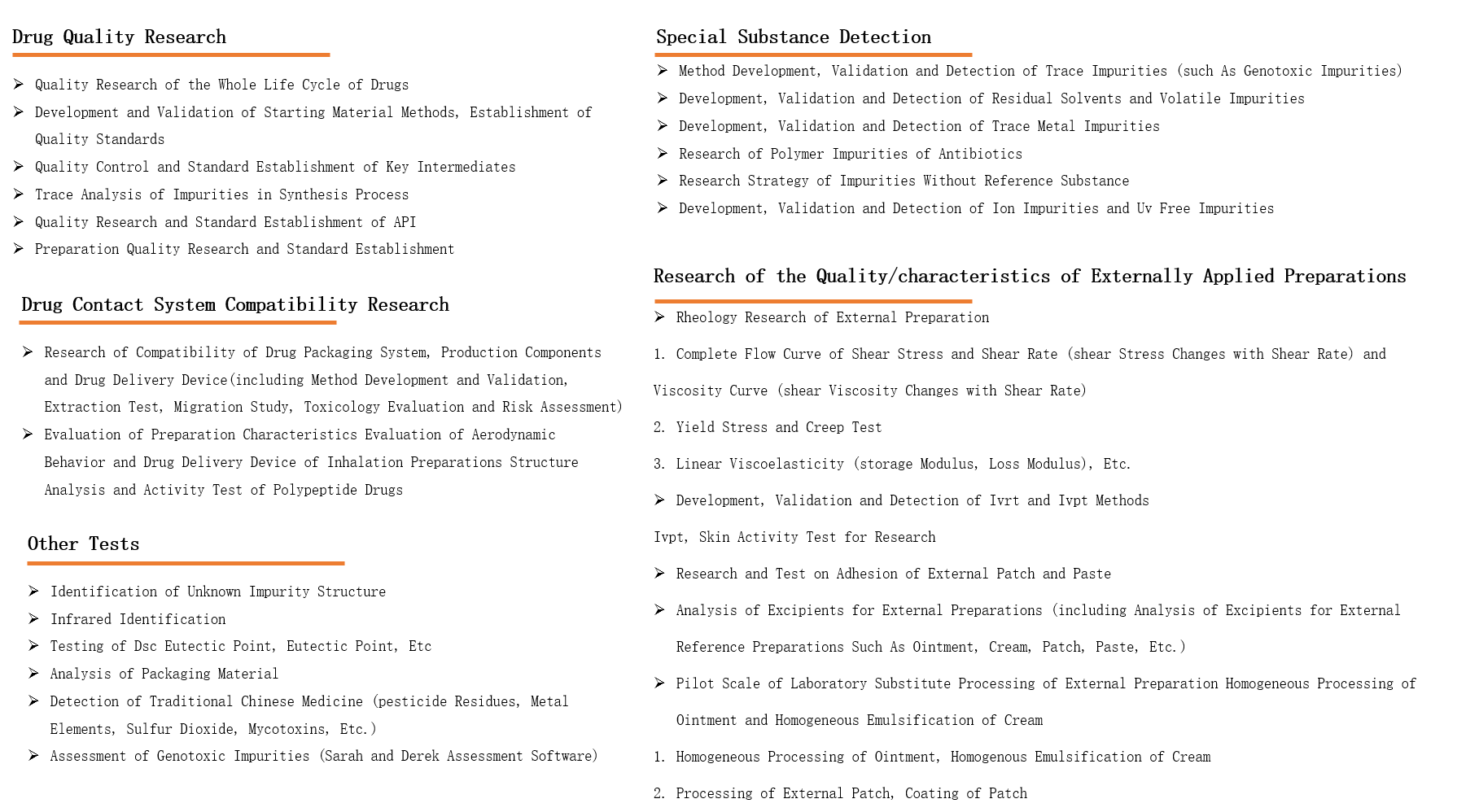

 Hot line:010-83057670
Hot line:010-83057670
 EN
EN








 010-83057670
010-83057670 Address:
Address: Marketing Department:
Marketing Department: Leadingpharm
Leadingpharm Pharm News
Pharm News 010-61006450
010-61006450
